Background:TP53 gene mutations lead to inactivation of the protein p53, causing genome instability, oncogenesis, and treatment resistance conferring an unfavorable prognosis. TP53 Y220C missense mutations in exon 6 (p.659A>G) are described as a frequent ‘hot spot’ mutation in solid tumors. A novel Y220C-targeted small molecule (PC14586) has shown preliminary efficacy and safety in patients with solid tumors (Dumbrava, E., ASCO 2022). No data has been reported about TP53 Y220C in hematologic malignancies to date. We set out to evaluate the clinicopathologic characteristics of TP53 Y220C in hematologic malignancies and particularly, myeloid neoplasms.
Methods: We identified all patients with hematologic neoplasms with TP53 Y220C mutations at University of Texas MD Anderson Cancer Center and Memorial Sloan-Kettering Cancer Center, detected by standard next generation sequencing assays, and focused on patients with myeloid malignancies; summarizing the patient characteristics, disease features and outcomes for patients with this specific TP53 mutation. Overall survival (OS) was assessed by the Kaplan-Meier method from date of diagnosis to date of death from any cause or date of last follow-up. Leukemia-free survival (LFS) was calculated from time to diagnosis of TP53 Y220C mutated myelodysplastic syndrome (MDS) to date of transformation to acute myeloid leukemia (AML) or death. Survival analysis was conducted only for newly diagnosed patients with available follow-up data.
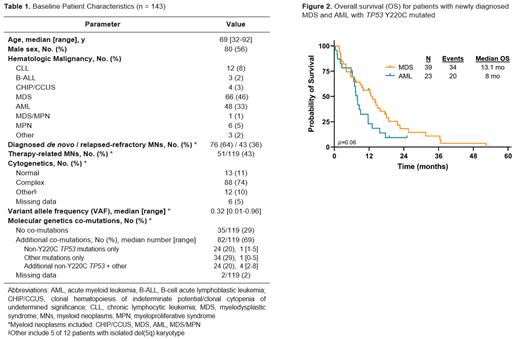
Results: We identified 143 patients (pts) with hematologic malignancies harboring a TP53 Y220C mutation. MDS and AML were the most frequent diagnoses (MDS, n=66; AML, n=48). Median age was 69 years (range, 32-92) and 56% of patients were male (Table 1). We then analyzed in more detail patients with MNs, including CHIP/CCUS, MDS, AML and MDS/MPN. Among those, in 76 (64%) pts the TP53 Y220C mutation was detected at initial diagnosis and in 43 (36%) pts, TP53 Y220C was identified in the relapsed/refractory setting. Therapy-related myeloid neoplasms (MNs) accounted for 51 of 119 pts (41%). Complex karyotype was frequent (n=88/119, 74%) in the setting of TP53 Y220C. Median TP53 Y220C variant allele frequency (VAF) was 32% (range, 1%-96%). Eighty-two patients (69%) had additional co-mutations: 24 (20%) pts had additional (non-Y220C) TP53 mutations only; 34 (29%) pts had only non- TP53 co-mutations (most frequently DNMT3A and TET2) and 24 (20%) pts had additional TP53 mutations in the setting of other co-mutations. With a median follow-up of 9 months, median OS for patients with newly diagnosed TP53 Y220C MDS (n=39) and AML (n=23) was 13.1 months (95% CI, 7.6 to 18.7) and 8 months (95% CI, 5.6 to 13.4), respectively ( p=0.06) (Figure 2). For the TP53 Y220C MDS cohort, AML transformation occurred in 13 (33%) patients, after a median of 14.3 months. Median LFS for these patients was 12.2 months (95% CI, 7.9 to 16.5). Modest and non-statistically significant differences in median LFS for MDS patients who did and did not receive stem cell transplant (SCT) were identified; MDS+SCT (n=12) 14.5 months (95% CI, 4 to 25) vs MDS+no-SCT (n=27) 9.3 months (95% CI, 4.6 to 14), p=0.18. Interestingly, four patients diagnosed with TP53 Y220C mutated CHIP/CCUS with a median VAF of 2% were identified, and with a median follow-up of 34.6 months, none have developed dysplastic changes or progression to MDS or AML at the time of cut off. Additional outcomes data will be forthcoming in the analysis of this cohort of patients with TP53 Y220C mutated myeloid neoplasms.
Conclusions:TP53 Y220C mutations are present in malignant blood disorders and are particularly more common in myelodysplastic syndromes and acute myeloid leukemia, with associated poor outcomes. Novel targeted therapies for this TP53 variant (Y220C) would be of interest for this patient population.
Disclosures
Loghavi:Astellas: Research Funding; Caris Diagnostics: Consultancy; Blueprint Medicine: Consultancy; Abbvie: Consultancy; Gerson Lehrman Group: Consultancy; QualWorld: Consultancy; Guidepoint: Consultancy; Recordati/ EUSA Pharma: Consultancy; Daiichi Sankyo: Consultancy; Amgen: Research Funding; Abbvie: Current equity holder in publicly-traded company. Goldberg:ADC Therapeutics: Research Funding; Prelude: Research Funding; Genentech: Consultancy; Aprea: Research Funding; Celularity: Research Funding; Pfizer: Research Funding; Aptose: Research Funding; DAVA Oncology: Honoraria; Abbvie: Consultancy, Research Funding; Astellas Pharma: Consultancy; Trillium: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; AROG: Research Funding. Issa:Kura Oncology: Consultancy, Research Funding; Syndax: Research Funding; Celgene: Research Funding; NuProbe: Consultancy; Merck: Research Funding; Novartis: Consultancy, Research Funding. Borthakur:Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy; Pacylex, Novartis, Cytomx, Bio Ascend:: Membership on an entity's Board of Directors or advisory committees; Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding. Kadia:Regeneron Pharmaceuticals: Research Funding; Pulmotect, Inc.: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Cure: Speakers Bureau; Novartis: Consultancy; Jazz Pharmaceuticals, Pfizer, Pulmotect, Inc, Regeneron Pharmaceuticals, SELLAS Life Sciences Group: Research Funding; Cyclacel: Research Funding; SELLAS Life Sciences Group: Research Funding; BMS: Consultancy, Research Funding; Pinotb-Bio: Consultancy; Daiichi Sankyo, Genentech, Inc., Genzyme, Jazz Pharmaceuticals, Liberum, Novartis, Pfizer, PinotBio, Inc, Pulmotect, Inc, Sanofi-Aventis, Servier: Consultancy; Agios: Consultancy; Janssen Research and Development: Research Funding; Amgen, Inc.: Research Funding; Liberum: Consultancy; Biologix, Cure, Hikma Pharmaceuticals: Speakers Bureau; Astellas Pharma Global Development: Research Funding; Genentech: Consultancy, Research Funding; Sanofi-Aventis: Consultancy; Celgene: Research Funding; Cellenkos Inc.: Research Funding; AstraZeneca: Research Funding; Glycomimetics: Research Funding; Hikma Pharmaceuticals: Speakers Bureau; Iterion: Research Funding; Ascentage Pharma Group: Research Funding; GenFleet Therapeutics: Research Funding; Servier: Consultancy; Delta-Fly Pharma, Inc.: Research Funding; Genzyme: Honoraria; AbbVie, Amgen, Inc, Ascentage Pharma Group, Astellas Pharma Global Development, Astex, AstraZeneca, BMS, Celgene, Cellenkos Inc, Cyclacel, Delta-Fly Pharma, Inc, Genentech, Inc., Genfleet, Glycomimetics, Iterion, Janssen Research and Development: Research Funding; Astex: Honoraria. Carter:PMV: Research Funding; Syndax: Research Funding; PinotBio: Research Funding; Revolution Medicines: Research Funding. Andreeff:Kintor Pharmaceutical: Research Funding; PMV: Research Funding. Stein:CTI Biopharma: Consultancy; Blueprint: Consultancy; Ono Pharma: Consultancy; Astellas: Consultancy; Foghorn: Consultancy; Syros: Consultancy; Aptose: Consultancy; Eisai: Research Funding; Bristol Myers Squib: Consultancy, Research Funding; Novartis: Consultancy; PinotBio: Consultancy; Janssen: Consultancy; Agios: Consultancy; Jazz: Consultancy; Menarini: Consultancy; Genentech: Consultancy; Genesis: Consultancy; Abbvie: Consultancy; Neoleukin: Consultancy; Gilead: Consultancy; Syndax: Consultancy; OnCusp: Consultancy; Daiichi: Consultancy; Calithera: Consultancy; Servier: Consultancy. DiNardo:AbbVie/Genentech: Honoraria; Astellas: Honoraria; ImmuniOnc: Honoraria; Servier: Honoraria; Notable Labs: Honoraria; Fogham: Honoraria; BMS: Honoraria; Novartis: Honoraria; Takeda: Honoraria; Schrödinger: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal